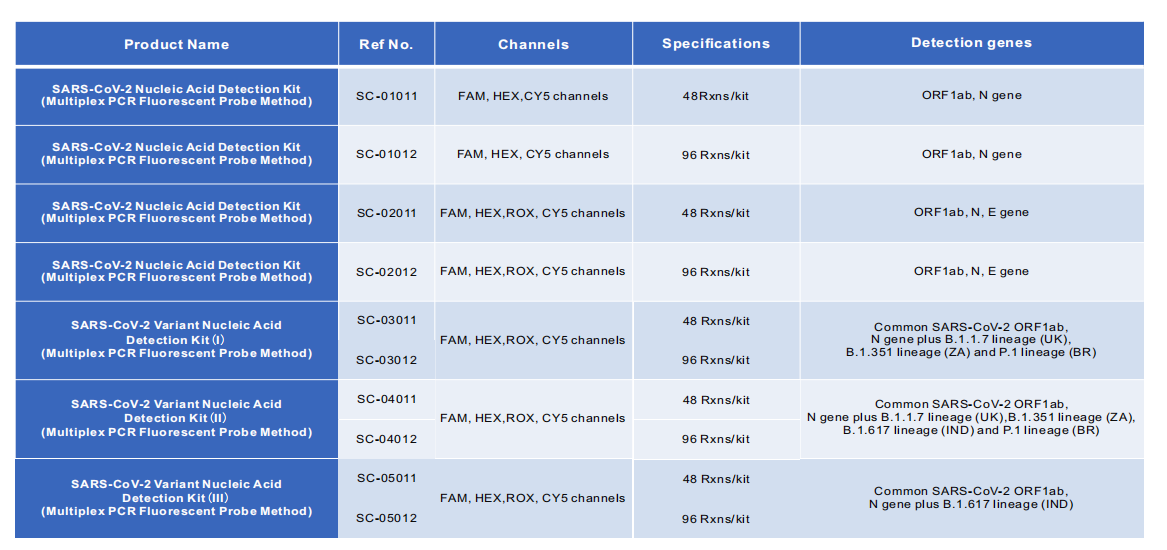
◮Applicable specimen types
Nasopharyngeal Swab or Oropharyngeal Swab
◮ Applicable instrument
ABI7500, Bio-Rad CFX96, Roche LightCycler 480, SLAN-96S
◮ Direct PCR
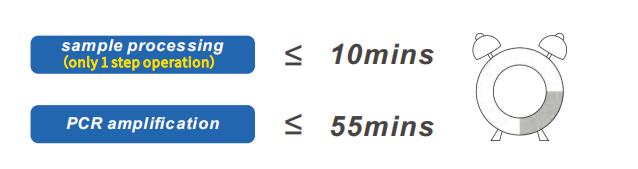
No need for nucleic acid extaction kit and System, completed 96 specimen in 1 hour or so.
◮ Low equipment requirement and highly flflexible use
Only need Real-Time PCR system.
◮ LoD and high sensitivity
Detection sensitivity can reach the lowest level as 500 copies/ml.
Method 1: Direct PCR method Method 2: Viral RNA Isolation + PCR Detection procedure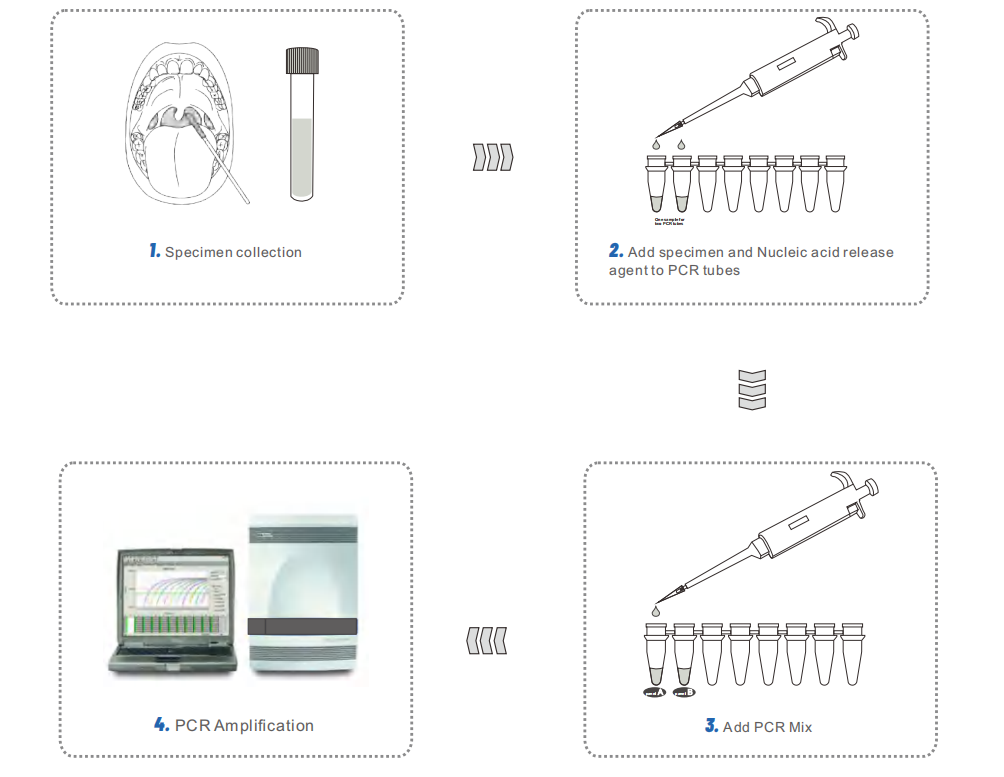 Sealed from light and stored at -20±5℃;
Sealed from light and stored at -20±5℃;  Shelf life
Shelf life | No. | Component | Amount | Main components | |||
| 48 Rxns | 96 Rxns | |||||
| 1 | Nucleic acid release agent | 1.4mL/tube | 2 tubes | 5.3 mL/bottle | 1 bottle | Surfactant |
| 2 | RNA protectant | 27 μL/tube | 1 tube | 53 μL/tube | 1 tube | RNase inhibitor |
| 3 | SARS-CoV-2 reaction solution | 800 μL/tube | 1 tube | 1600 μL/tube | 1 tube | Primer, probe, reaction buffer, dNTP |
| 4 | SARS-CoV-2 enzyme mixture | 80 μL/tube | 1 tube | 160 μL/tube | 1 tube | Hot start Taq enzyme, M-MLV enzyme |
| 5 | SARS-CoV-2 positive control | 100 μL/tube | 1 tube | 100 μL/tube | 1 tube | Recombinant plasmid containing target fragment, RNA |
| 6 | SARS-CoV-2 negative control | 1200 μL/tube | 1 tube | 1200 μL/tube | 1 tube | TE buffer |
 1 yearMethod 1: Direct PCR method using Sample Release Agent
1 yearMethod 1: Direct PCR method using Sample Release Agent This kit uses Real-time RT PCR technology (rRT-PCR) for qualitative detection of SARS-CoV-2 nucleic acids in human nasopharyngeal or oropharyngeal swab samples.
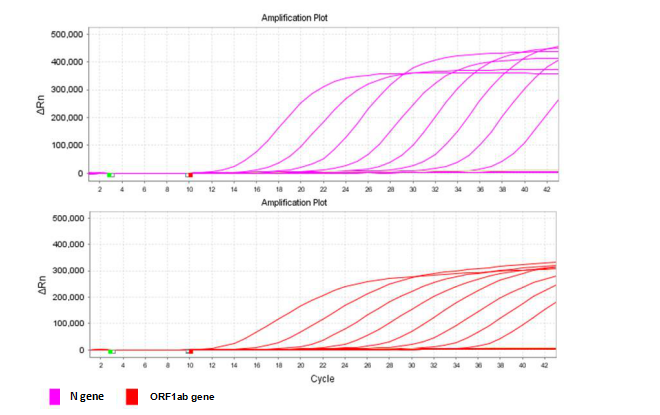
This primer and probe sets are designed for the detection of the conserved sequences of SARS-CoV-2 (ORF1ab gene and N gene). The kit contains an Internal Control that is used to assess specimen quality.
Real-Time PCR technology utilizes polymerase chain reaction (PCR) for the amplification of specific target
sequences and target specific probes for the detection of the amplified RNA. The probes are labelled with
fluorescent reporter and quencher dyes.
